Our mission is to be the very first biotechnology company offering commercially attractive, economically viable, and affordable personalized T cell therapies for large populations.
Clinical-Stage Specialty Immunotherapy Company
Founded in 2020, Tevogen Bio is built on a business philosophy of sustainable commercial success through innovation of affordable personalized immunotherapies for large patient populations. To address this unmet need we are harnessing one of nature’s most powerful immunological weapons, the genetically unmodified CD8+ cytotoxic or killer T cells, to develop off-the-shelf, precision T cell therapies for the treatment of infectious diseases, cancers, and neurological disorders.
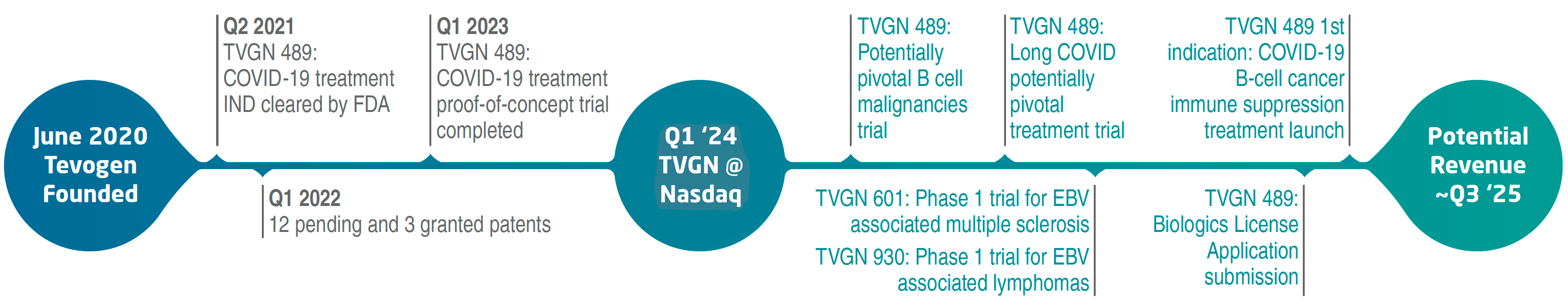
Tevogen’s Path to Revenue and Serving Patients
On February 15th, 2024, Tevogen Bio Holdings began public trading on the Nasdaq exchange under the ticker ‘TVGN’. Since inception, our achievements have underscored the potential of Tevogen’s disruptive biopharma business model to achieve a shorter path to revenue compared to the industry average.
Key intellectual property assets are wholly owned by Tevogen Bio and are not subject to any third-party licensing agreements. These assets include three granted patents and twelve pending patents, two of which are related to artificial intelligence.

Tevogen’s Leadership
Along with significant combined experience, our leadership has experience across all sectors of the healthcare ecosystem. Our leadership is dedicated to charting the next frontier of healthcare, where disruptive science and business models pave the way to a healthier future for all.

Ryan Saadi, MD, MPH
Chief Executive Officer

Neal Flomenberg, MD
Chief Scientific Officer
and Global R&D Lead

Kirti Desai, CPA
Chief Financial Officer
Board of Directors:
- Curtis Patton, PhD – Professor Emeritus, Yale
- Susan Podlogar, MBA – CHRO, MetLife
- Lindee Goh, PhD – Partner, Tapestry Networks
- Jeffrey Feike, MPH – Former Hospital President
- Victor Sordillo, PE, CSP, MBA – Managing Director, Risk Advisory Services, Verita CSG, Inc.
- Suren-Ajjarapu – CEO, Trxade Health, Inc.
Tevogen’s T Cell Therapy Platform – ExacTcellTM
ExacTcell focuses on the selection and expansion of naturally occurring, genetically unmodified CD8+ cytotoxic t lymphocytes to target multiple, distinct, preselected antigens present only on virus-infected or malignant cells and to kill those cells. This is in contrast with both autologous and allogeneic CAR-T platforms, which target antigens present on both healthy and diseased cells and require genetic modification of the T cells. We believe our ExacTcell platform represents a scientific breakthrough, offering potential for off-the-shelf drugs in virology, oncology, and neurology.

Tevogen’s Disruptive Science

Tevogen.ai Aims to Enhance Patient Access by Utilizing Artificial Intelligence-powered Tools
Target Detection: We are exploring ways to deploy AI-powered target detection to further accelerate our product development pace, both either internally or in collaboration with others.
Reducing Failure Rates: AI could use data patterns to foresee potential adverse drug reactions early on, potentially averting costly trial failures. It might also flag efficacy concerns, guiding timely adjustments to enhance the probability of success.
Optimizing Clinical Trials: AI algorithms could analyze data to identify patients who would be most likely to respond to the investigational therapy.
Tevogen’s Research Pipeline

For more information, please contact Tevogen Investor Relations
Forward-Looking Statements
This presentation contains certain forward-looking statements, including without limitation statements relating to: expectations regarding the healthcare and biopharmaceutical industries; Tevogen’s development of, the potential benefits of, and patient access to its product candidates for the treatment of infectious diseases, cancer and neurological disorders, including TVGN 489 for the treatment of COVID-19 and Long COVID; Tevogen’s ability to develop additional product candidates, including through use of Tevogen’s ExacTcell platform; the anticipated benefits of ExacTcell; expectations regarding Tevogen’s future clinical trials; Tevogen’s manufacturing plans; and Tevogen’s ability to generate revenue in the future. Forward-looking statements can sometimes be identified by words such as “may,” “could,” “would,” “expect,” “possible,” “potential,” “goal,” “opportunity,” “project,” “believe,” “future,” and similar words and expressions or their opposites. These statements are based on management’s expectations, assumptions, estimates, projections and beliefs as of the date of this presentation and are subject to a number of factors that involve known and unknown risks, delays, uncertainties and other factors not under the company’s control that may cause actual results, performance or achievements of the company to be materially different from the results, performance or other expectations expressed or implied by these forward-looking statements.
These factors include, but are not limited to: (i) the effect of the recent business combination with Semper Paratus Acquisition Corporation (the “Business Combination”) on Tevogen’s business relationships, operating results, and business generally; (ii) the outcome of any legal proceedings that may be instituted against Tevogen related to the Business Combination; (iii) changes in the markets in which Tevogen competes, including with respect to its competitive landscape, technology evolution, or regulatory changes; (iv) changes in domestic and global general economic conditions; (v) the risk that Tevogen may not be able to execute its growth strategies or may experience difficulties in managing its growth and expanding operations; (vi) the risk that Tevogen may not be able to develop and maintain effective internal controls; (vii) costs related to the Business Combination and the failure to realize anticipated benefits of the Business Combination; (viii) the failure to achieve Tevogen’s commercialization and development plans, and identify and realize additional opportunities, which may be affected by, among other things, competition, the ability of Tevogen to grow and manage growth economically and hire and retain key employees; (ix) the risk that Tevogen may fail to keep pace with rapid technological developments to provide new and innovative products and services or make substantial investments in unsuccessful new products and services; (x) the ability to develop, license or acquire new therapeutics; (xi) that Tevogen will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (xii) the risk of regulatory lawsuits or proceedings relating to Tevogen’s business; (xiii) uncertainties inherent in the execution, cost, and completion of preclinical studies and clinical trials; (xiv) risks related to regulatory review, and approval and commercial development; (xv) risks associated with intellectual property protection; (xvi) Tevogen’s limited operating history; and (xvii) those factors discussed in Tevogen’s filings with the SEC and that that are contained in the Proxy Statement/Prospectus relating to the Business Combination.
You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Tevogen undertakes no obligation to update any forward-looking statements, except as required by applicable law.

© 2024 Tevogen Bio. All Rights Reserved.
