TCTL Trial
Third-Party Covid-19-Specific Cytotoxic T Lymphocytes for the Treatment of Elderly and High-Risk Patients with Covid-19
Actual Study Completion Date: January 19, 2023
An Open-label single center two arm study to assess the safety and efficacy of SARS-CoV-2-specific T cells when given as treatment to adult patients (age ≥ 18 years) with a newly diagnosed SARS-CoV-2 infection. This immunologic treatment is aimed at patients, who are at high risk of progression due to their advanced age, or other underlying health conditions.
Peer Review
Safety and Feasibility of Third-Party Cytotoxic T Lymphocytes for High-Risk Patients with Covid-19
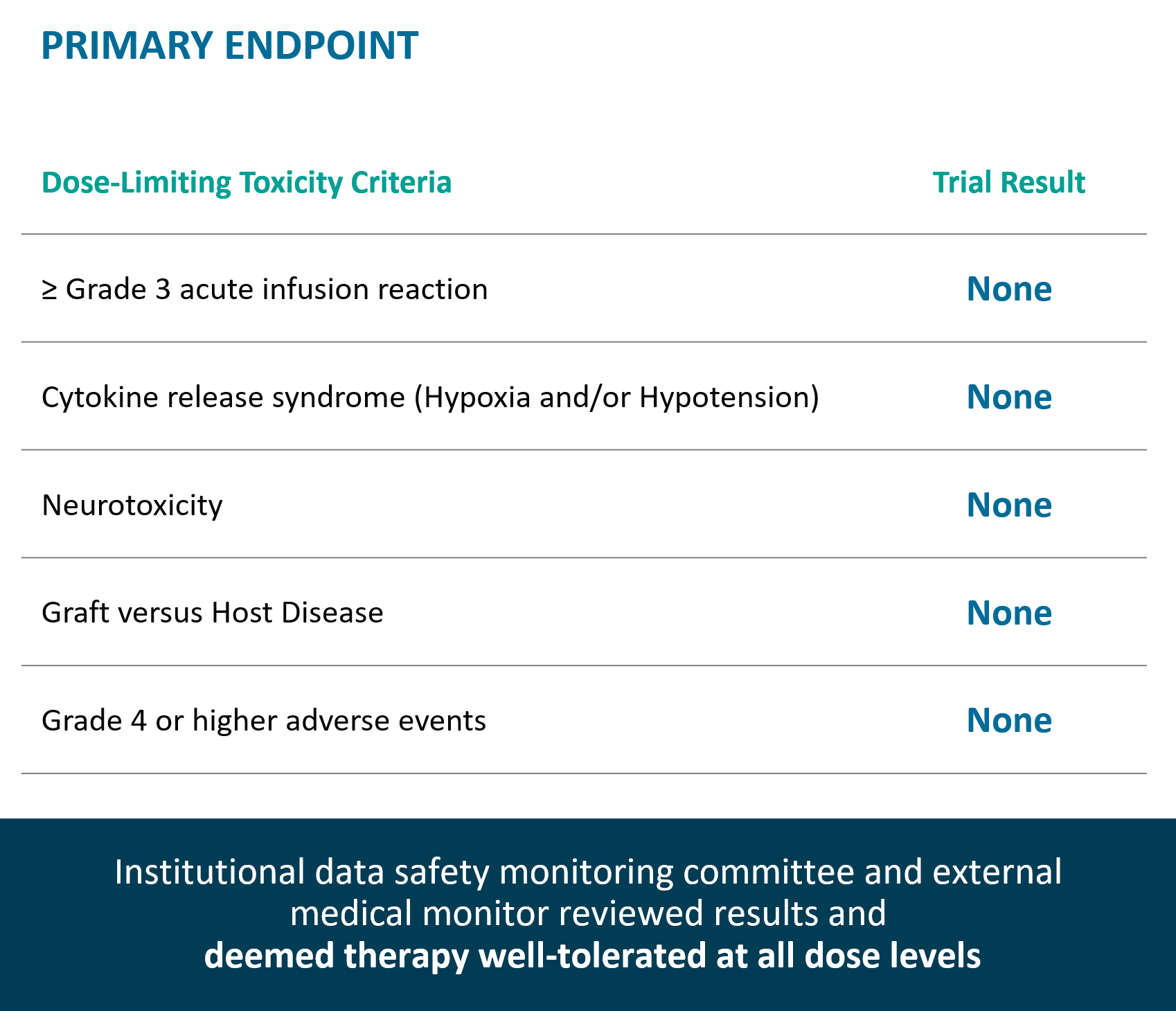
Key Observations
Therapy well-tolerated at all dose levels, no dose limiting toxicities observed.
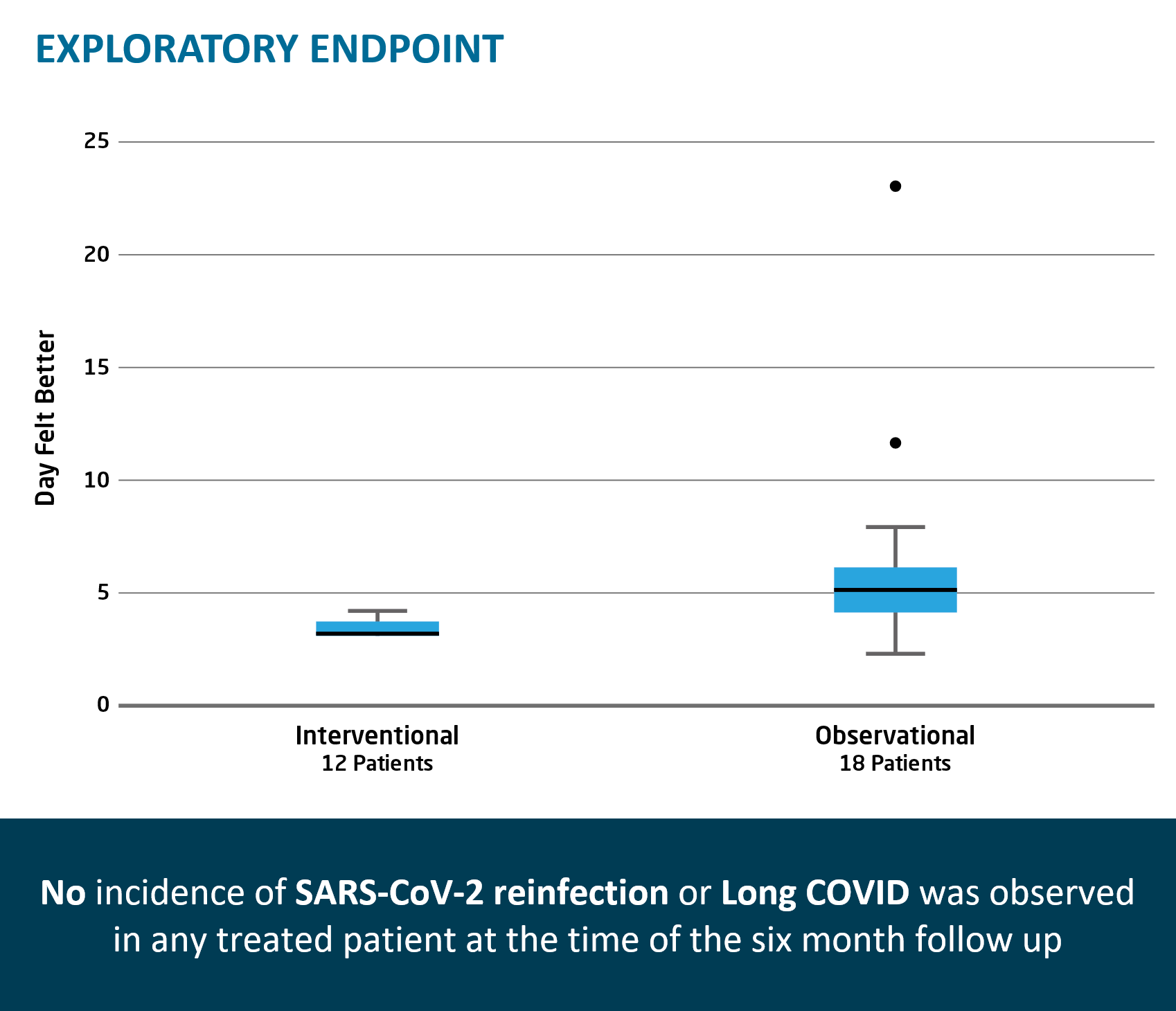
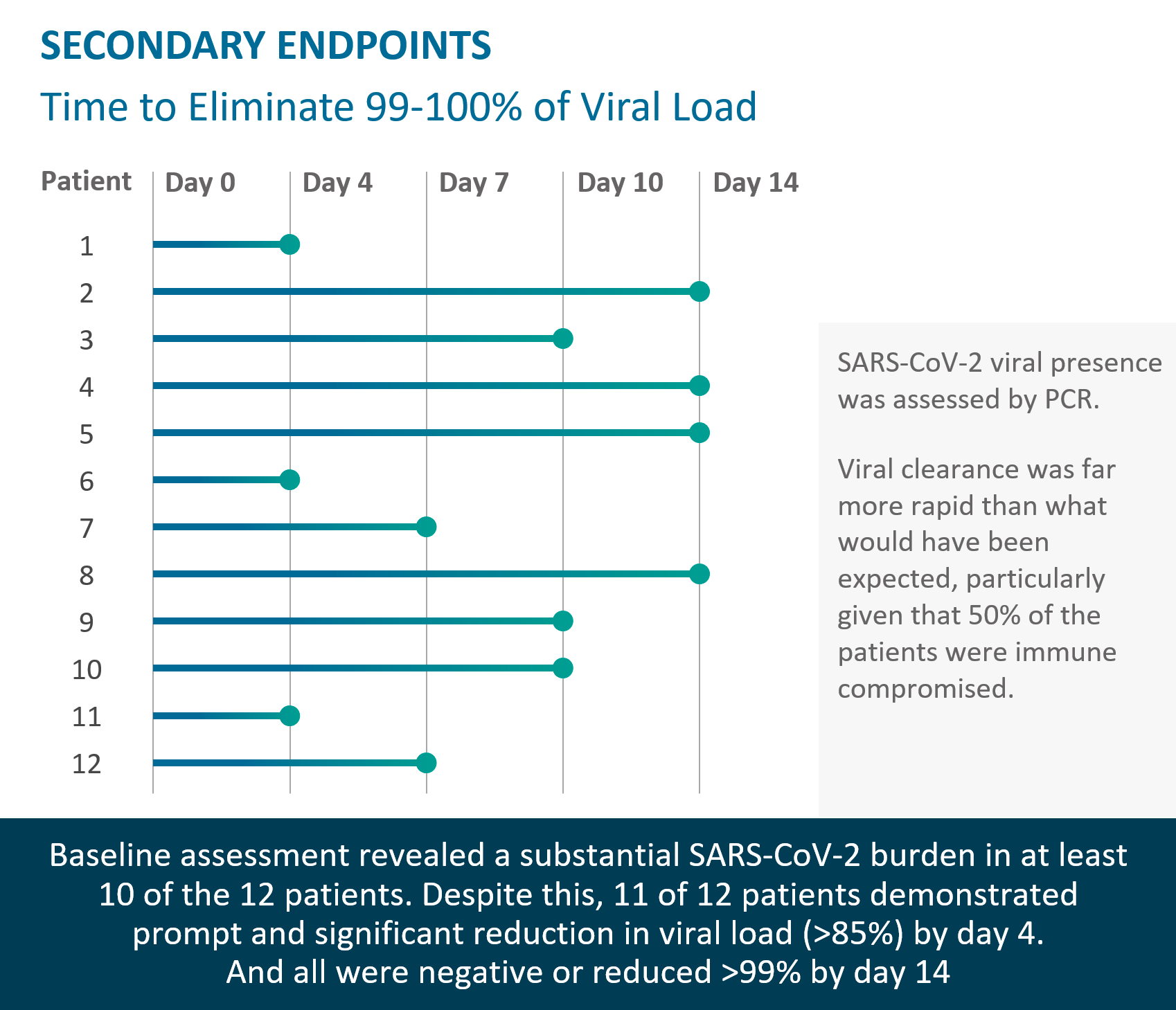
Rapid viral load reduction after TVGN 489 treatment; documented persistence of TVGN 489

For additional information about the study,
call 267-239-6281 or e-mail clinicaltrials@tevogen.com
COVID-19 and Tevogen’s Investigational Therapy

© 2026 Tevogen Bio. All Rights Reserved.
